Building with a Programmable Molecular Robot
‘Stereodivergent synthesis with a programmable molecular machine’ Salma Kassem, Alan T. L. Lee, David A. Leigh, Vanesa Marcos, Leoni I. Palmer and Simone Pisano, Nature, 549, 374-378 (2017). Full Article.
[Image credit: Stuart Jantzen, www.biocinematics.com]
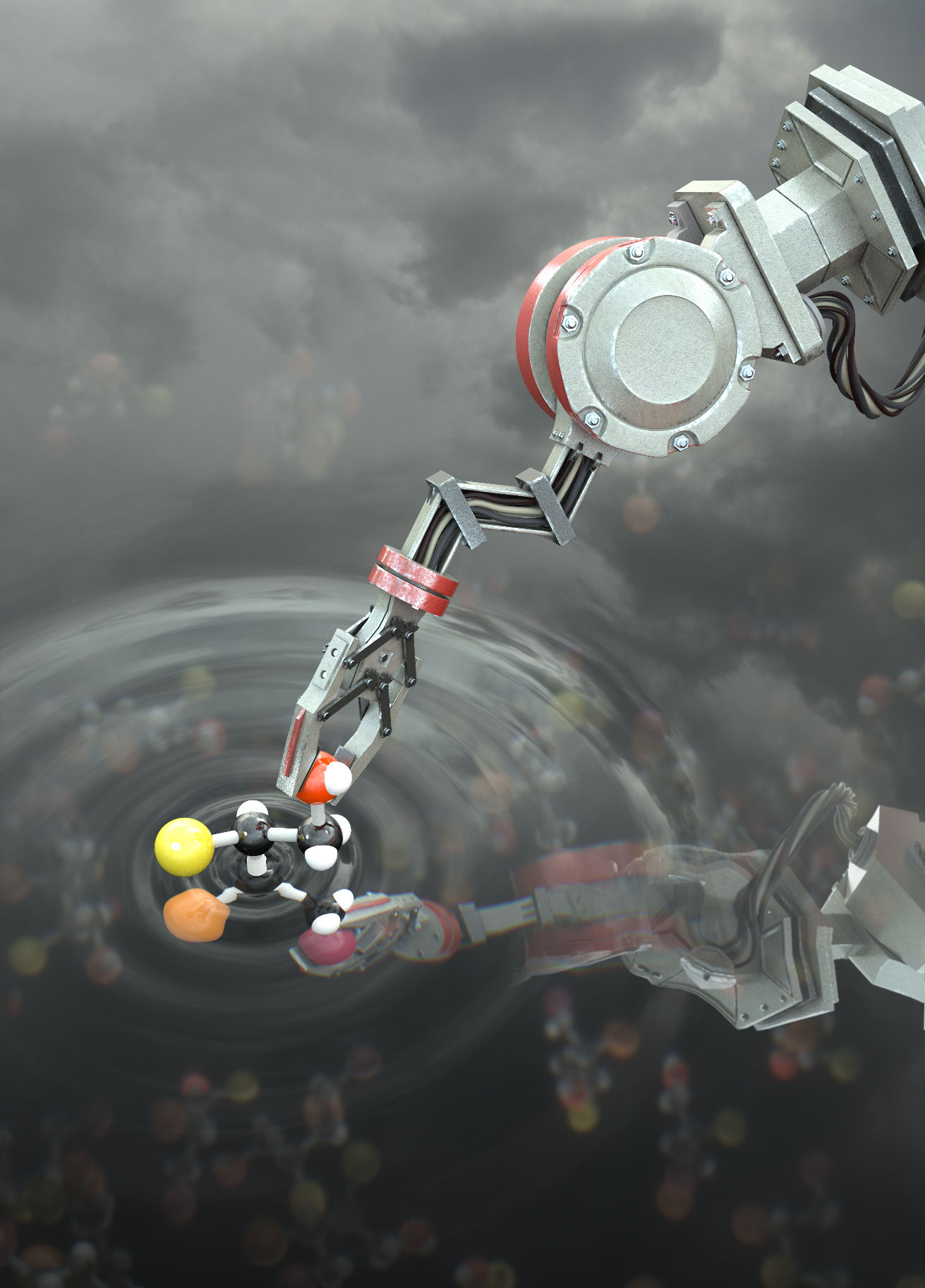
A ‘molecular assembler’ is a hypothetical futuristic concept for a ‘nanobot’ that is ‘able to guide chemical reactions by positioning reactive molecules with atomic precision’ (Figure 1a),1 originally proposed2 by K. Eric Drexler and inspired by Richard P. Feynman’s lecture ‘There’s Plenty of Room at the Bottom’.3 It has been convincingly argued4-6 that molecular assemblers that manipulate individual atoms or highly reactive clusters of atoms with angstrom precision are unlikely to be realised because of the so-called ‘sticky fingers’ and ‘fat fingers’ problems. However, biological molecular machines routinely position (rather less reactive) substrates in order to direct chemical reaction sequences, from sequence-specific synthesis by the ribosome7 to polyketide synthases,8-10 where tethered molecules are passed from active site to active site in multi-enzyme complexes (Figure 1b).

Now scientists at the University of Manchester have developed a molecular robot that moves a substrate between different activating sites in order to achieve different product outcomes from chemical synthesis.11 The molecular robot can be programmed to selectively produce, in a sequential one-pot operation, any one of four possible diastereoisomers from a tandem reaction process, including compounds that cannot be made12 through conventional iminium-enamine organocatalysis. The molecular machine design is based on a previous molecular robot that could transport a cargo in either direction between two sites on a molecular platform.13 In the new design the platform sites are organocatalysts of different chirality and the cargo is a substrate that is moved by the robotic arm between the two sites to carry out successive chemical reactions. Depending on which side the substrate is held for each reaction, a different stereoisomer of the product is formed (Figure 2). The reaction sequences are carried out in one pot and the robot can be programmed to produce selectively each isomer of the product by controlling the switch-state prior to each reaction of the substrate. Each molecular robot manipulates a single substrate molecule, but the process is massively paralleled with more than a billion billion (i.e., 1018) molecular robots operated simultaneously by the scientists. Future generations of programmable molecular machines may play significant roles in chemical synthesis and molecular manufacturing.
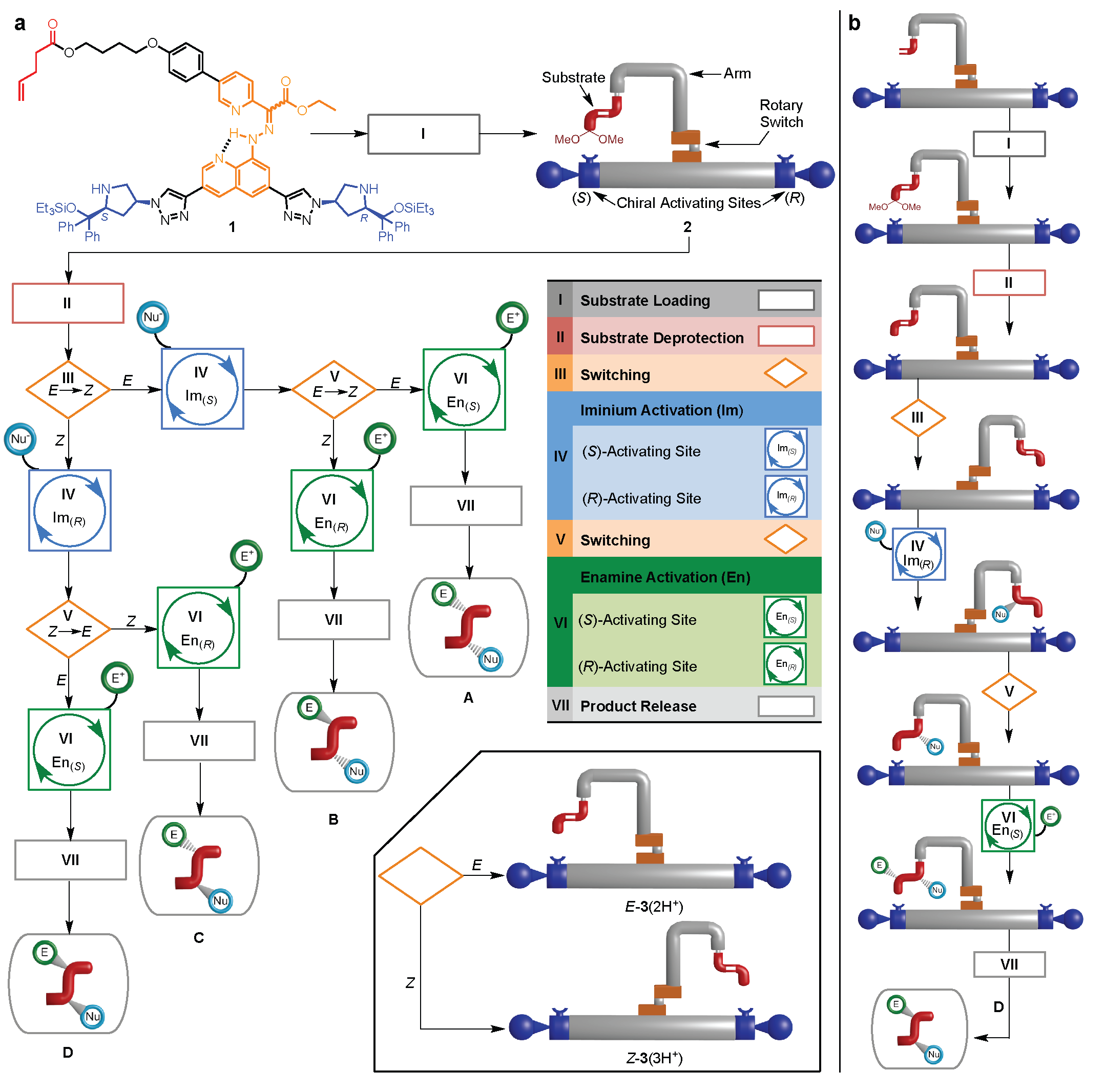
Figure 2. Programmable operation of a molecular machine that synthesizes different products by moving a substrate between different activating sites. a, Flowchart showing the programmable synthesis of any one of four stereoisomers by the molecular robot. b, The molecular robot’s response to command inputs in program D.
Using a thiol nucleophile for the first reaction (iminium activation of the substrate by the machine) and an electron-poor alkene electrophile for the second reaction (enamine activation of the substrate by the machine) any of the four possible products can be selectively made by the robot in one pot through different programing (Figure 3 and Figure 4). However, the machine does not behave exactly as designed, the relative stereochemistry of the second reaction is controlled by the switch-state rather than the chirality of the organocatalyst as originally envisioned. This illustrates the challenge and complexity of designing multicomponent molecular machines with interactive parts.14
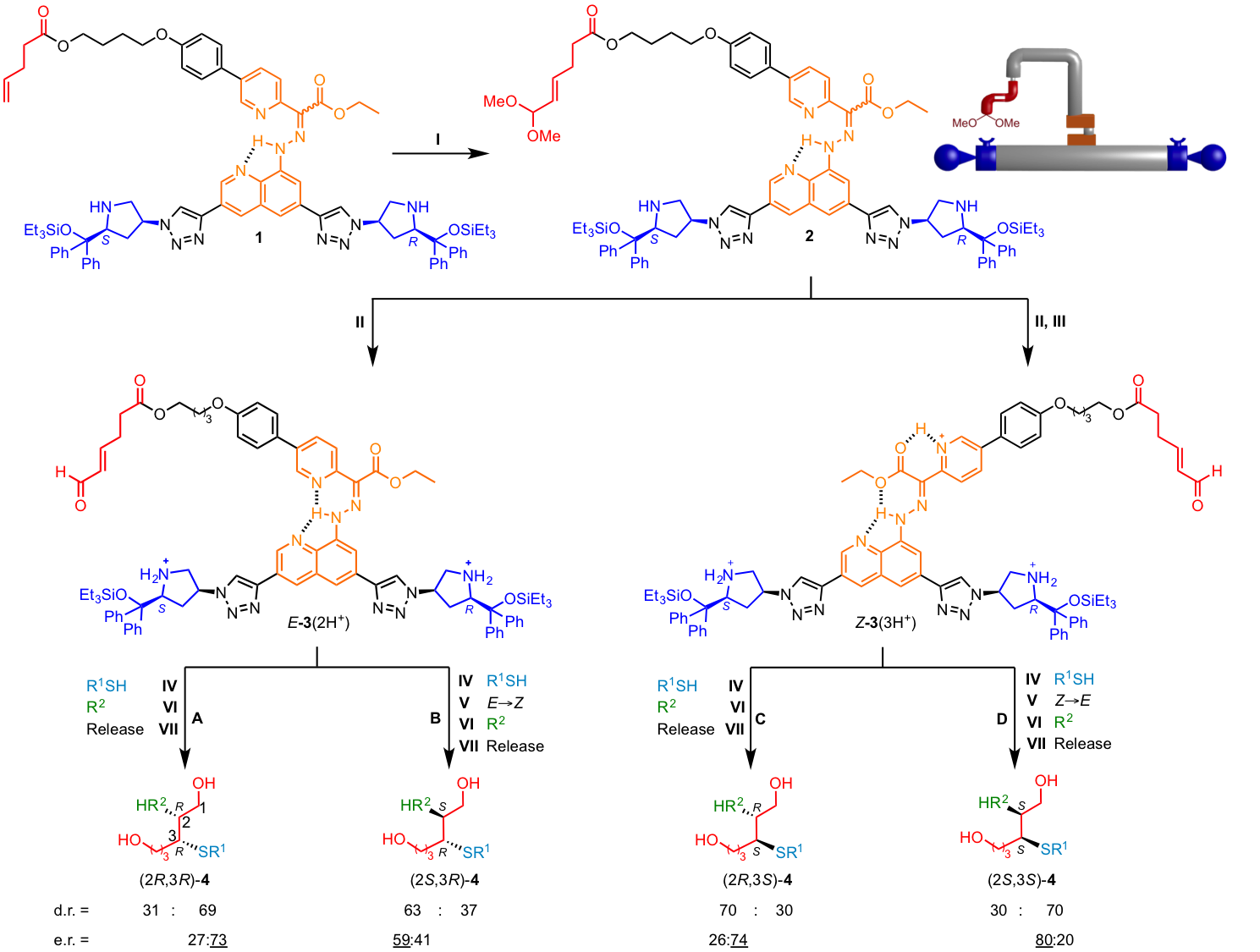
Figure 3. Programmable synthesis of each possible stereoisomer of the product from one-pot tandem iminium-enamine promoted reaction sequences by the molecular machine. R1 is (CH2)2(CF2)7CF3. R2 is H2C=C(SO2Ph)2.
This first generation machine augurs well for the development of small-molecule robots that can be programmed to manipulate substrates to control synthesis in a form of mechanosynthesis (that is, the use of mechanical constraints to direct reactive molecules to specific molecular sites2), in a manner reminiscent of the way that molecular construction is carried out in biology.
Figure 4. Stylised ’nanopunk’ representation of the programmed operation of the molecular assembler. [Video credit: Stuart Jantzen, www.biocinematics.com] [Click here to download the HD version of the video].
References
[1] Drexler, K. E. in ‘Point-Counterpoint’, Chem. & Eng. News 81(48), 37-42 (2003).
[2] Drexler, K. E. Engines of Creation: The Coming Era of Nanotechnology (Anchor Books, New York, 1986).
[3] Feynman, R. P. There’s plenty of room at the bottom. Eng. Sci. 23(5), 22-36 (1960).
[4] Smalley, R. E. Of chemistry, love, and nanobots. Sci. Am. 285(3), 76-77 (2001).
[5] Whitesides, G. M. The once and future nanomachine. Sci. Am. 285(3), 78-83 (2001).
[6] Jones, R. A. L. Soft Machines: Nanotechnology and Life (Oxford Univ. Press, 2004).
[7] Yonath, A. Hibernating bears, antibiotics, and the evolving ribosome (Nobel Lecture). Angew. Chem. Int. Ed. 49, 4340–4354 (2010).
[8] Maier, T., Leibundgut, M. & Ban, N. The crystal structure of a mammalian fatty acid synthase. Science 321, 1315–1322 (2008).
[9] Brignole, E. J., Smith, S. & Asturias, F. J. Conformational flexibility of metazoan fatty acid synthase enables catalysis. Nat. Struct. Mol. Biol. 16, 190–197 (2009).
[10] Chan, D. I. & Vogel, H. J. Current understanding of fatty acid biosynthesis and the acyl carrier protein. Biochem. J. 430, 1–19 (2010).
[11] Kassem, S., Lee, A. T. L., Leigh, D. A., Marcos, V., Palmer, L. I. & Pisano, S. Stereodivergent synthesis with a programmable molecular machine. Nature doi:10.1038/nature23677 (2017).
[12] Krautwald, S. & Carreira, E. M. Stereodivergence in asymmetric catalysis. J. Am. Chem. Soc. 139, 5627–5639 (2017).
[13] Kassem, S., Lee, A. T. L., Leigh, D. A., Markevicius, A. & Solà, J. Pick-up, transport and release of a molecular cargo using a small-molecule robotic arm. Nat. Chem. 8, 138–143 (2016).
[14] Lewandowski, B. et al. Sequence-specific peptide synthesis by an artificial small-molecule machine. Science 339, 189–193 (2013).
